Pivotal data on glofitamab, a potential first-in-class CD20xCD3 T-cell engaging bispecific antibody in heavily pre-treated patients with aggressive lymphoma, will be presented as part of our industry-leading hematology portfolio
Further studies exploring broad genomic testing to support informed treatment decisions for patients and advance cancer care approaches will be presented
Genentech, a member of the Roche Group (SIX: RO, ROG; OTCQX: RHHBY), today announced that new data from clinical trials of 18 approved and investigational medicines across more than 20 cancer types will be presented at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, which will be held June 3-7, 2022. Genentech and its partners will present clinical studies across medicines, comprehensive genomic tests, and real-world data at this year’s meeting.
“At ASCO this year, progress from our portfolio, partnerships and collaborations showcase our commitment to advance innovation in cancer care,” said Levi Garraway, M.D., Ph.D., chief medical officer and head of Global Product Development. “We’re especially pleased to present data from our broad hematology portfolio, including pivotal data for glofitamab, a potential first-in-class bispecific antibody that may improve the lives of people with heavily pre-treated aggressive lymphoma.”
Focusing on improving outcomes in non-Hodgkin lymphoma
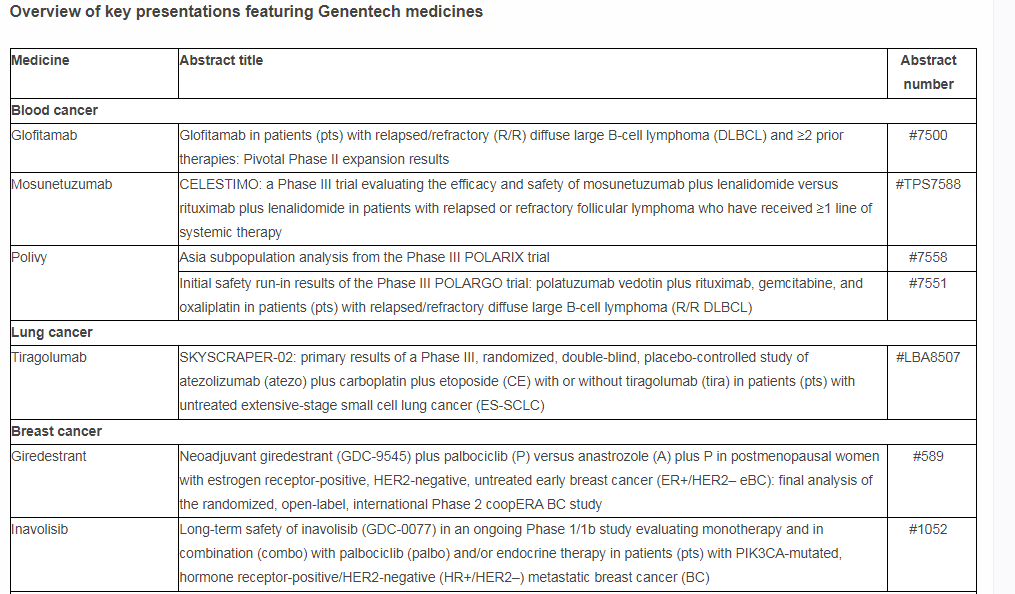
New and updated data in non-Hodgkin lymphoma will be presented at ASCO. This includes pivotal data from the Phase II NP30179 study investigating glofitamab, an investigational CD20xCD3 T-cell engaging bispecific antibody, in heavily pre-treated patients with diffuse large B-cell lymphoma (DLBCL). DLBCL is an aggressive form of lymphoma, where as many as 40% of patients will relapse, at which point treatment options are limited and survival is shortened. Glofitamab is part of Genentech’s broad bispecific antibody development program, which may offer a new immunotherapy-based approach to tackle a range of blood cancers. It is being investigated in several clinical trials including the STARGLO Phase III study, evaluating glofitamab in combination with gemcitabine and oxaliplatin (GemOx) versus MabThera®/Rituxan® (rituximab) in combination with GemOx in autologous stem-cell transplant ineligible relapsed or refractory DLBCL. In addition, key findings from an analysis of the Asia subpopulation from the pivotal Phase III POLARIX study investigating Polivy® (polatuzumab vedotin) in combination with MabThera/Rituxan plus cyclophosphamide, doxorubicin and prednisone (R-CHP) in people with newly diagnosed DLBCL will be featured. Polivy plus R-CHP is the first treatment regimen to significantly improve outcomes in previously untreated DLBCL in more than 20 years, potentially transforming treatment for people with this disease.
Driving innovation in personalized cancer care
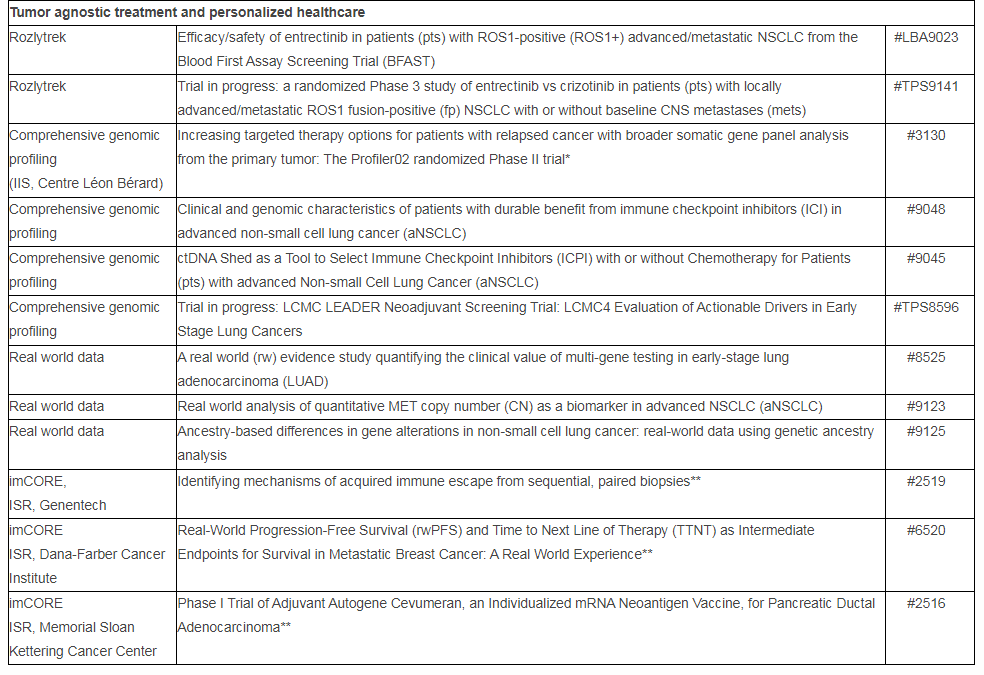
More than 20 new pieces of research from partnerships with Foundation Medicine will be presented, which continue to support innovation as well as progress in personalized cancer care. This includes new data from the Phase II Profiler02 study,* which investigates the use of a comprehensive genomic profiling testing panel from Foundation Medicine, with the aim of informing possible treatment decisions for patients based on their tumor’s unique genomic information.
Data from the imCORE network
Additionally, three abstracts from the Immunotherapy Centers Of Research Excellence (imCORE) Network will be presented at ASCO: a Phase I study** investigating autogene cevumeran (an mRNA-based individualized neoantigen-specific immunotherapy [iNeST]***) in the adjuvant setting of pancreatic ductal adenocarcinoma; a data mining study** evaluating intermediate endpoints for survival in metastatic breast cancer in the real-world setting; and a study identifying mechanisms of acquired resistance to immune checkpoint blockade.**
imCORE is an academic-industry network for scientific collaboration. Established by Genentech and connecting experts from 26 leading institutions around the globe, imCORE is committed to advancing and accelerating cancer immunotherapy research. imCORE is an example of Genentech’s dedication to collaborating with the global cancer community to further understand cancer biology and immunology, help inform the development of potential future treatment, and transform patients’ lives.
Genentech’s data presented at ASCO will feature its efforts to drive innovation and commitment to health equity through delivery of pioneering medicines and personalized cancer care that together improve outcomes for every patient while reducing the cost to society, inclusive clinical trials that remove barriers to participation, partnerships that multiply our ability to address challenges in cancer care, and bringing innovation into earlier stages of disease to maximize a chance of cure.
* IIS, investigator-initiated study
** ISR, institution-sponsored research
*** jointly developed by Genentech and BioNTech
For more such updates and perspectives around Digital Innovation, IoT, Data Infrastructure, AI & Cybersecurity, go to AI-Techpark.com.

